What Is the Best Lewis Structure for Cs2
Third structure is best Lewis str View the full answer Transcribed image text. Looking at the CS2 Lewis structure CS2 is composed of one carbon atom and two sulfur atoms.
The sulfur atom is required two electrons to complete the octet of sulfur atoms.

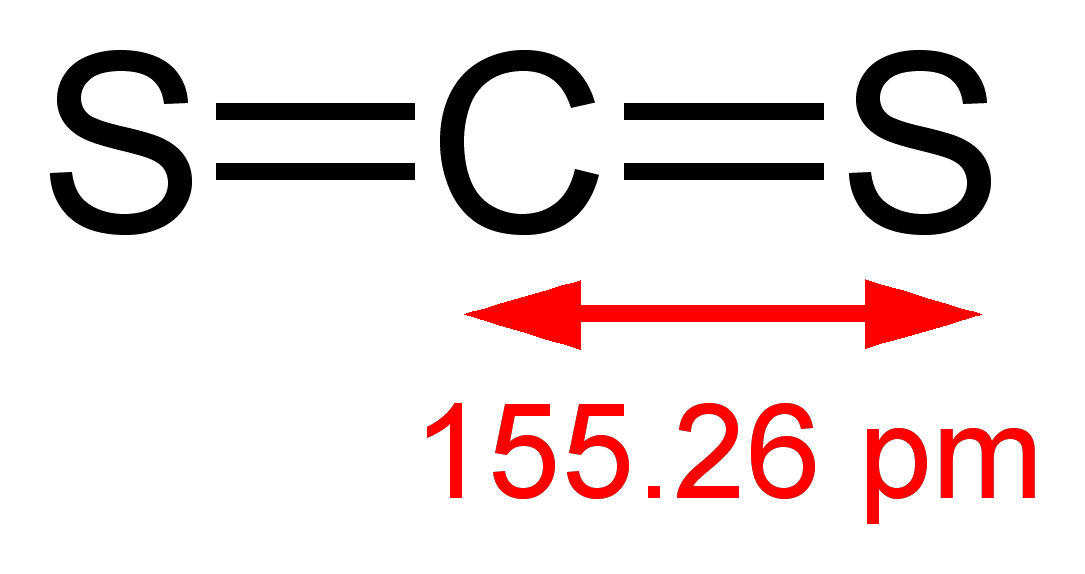
. Double bonds act as one electron pair to help determine electron-pair geometries of molecules according to VESPR. The general formula for linear geometry is AX2 and thus CS2 shows linear geometry. This species has its three atoms bonded sequentially in the following fashion.
Place the following in order of increasing dipole moment. Who are the experts. Choose the best Lewis structure for SO4 2-Draw the best Lewis structure for Cl3-.
The structure determines how sharing of the valence electrons is taking place and whether a single double or triple bond is forming. View the full answer. This molecule has two Sulphur atoms and one Carbon atom.
Carbon is the least electronegative atom and goes in the center of this structure. It is an ambident nucleophile in nucleophilic substitution. Question 4 2 points Which of following structures is the best Lewis structure for CS2.
The hybridization of the atoms in this idealized Lewis structure is given in the table below. The Lewis structure for CS2 is. The lewis structure for CS2 is.
Which of these covalent bonds is the most polar. Try to draw the CS 2. The central carbon atom will form double bonds with the two sulfur atoms.
The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in order to fill the octet of Carbon. With this information molecular. Sulfur contains six outermost valence electrons which means it contains six electrons in its outermost shell whereas carbon has four outermost electrons.
NCO- Lewis Structure Hybridization Molecular Geometry and Shape. A step-by-step explanation of how to draw the CS2 Lewis Dot Structure Carbon disulfideFor the CS2 structure use the periodic table to find the total numbe. Carbon is the least electronegative atom and goes in the center of this structure.
Carbon is the least electronegative atom and goes in the center of this structure. Choose the best Lewis structure for OCl2. The electron-pair geometry of CS2 is linear because the Lewis structure is SCS.
What is the correct formula for this compound. Drawing the Lewis Structure for CS 2 Sulfur Trioxide CS 2 is sometimes used to fumigate railroad cars and grain elevators. CS2 Lewis Structure Hybridization Polarity and Molecular Shape.
The electron-pair geometry of CS2 is linear because the Lewis structure is SCS. The best place to start when trying to figure out a molecules geometry is its Lewis structure. To understand the hybridization molecular geometry and the polarity of this molecule it is essential to under its Lewis structure.
Is CS2 polar or nonpolar. Carbon atoms have the least electronegative charge. There are 16 valence electrons for the CS2 Lewis structure.
The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in order to fill the octet of Carbon. Choose the best Lewis structure for ICl5. Best Lewis Structure The Lewis structure that is closest to your structure is determined.
Hybridization in the Best Lewis Structure. Carbon disulfide CS2 will have a total of 16 valence electrons 4 from the carbon atom and 6 from each of the two sulfur atoms. The core atom is carbon which is flanked by two sulfur atoms.
A valid Lewis structure of CS3 cannot be drawn. The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in. Am I correct in this assumption.
There are 16 valence electrons for the CS2 Lewis structure. Carbon is the least electronegative atom and goes in the center of this structure. As the hybridization of CS2 is sp hybridization the Carbon atom is in center bonding with two sulfur atoms forms the bond angle of 180 degrees making the molecular geometry of CS2 molecule linear.
My answer was that a valid structure cannot be drawn because CS3 displays the property of resonance. Experts are tested by Chegg as specialists in their subject area. The Lewis structure for CS2 requires you have double bonds between the Carbon C and Sulfur atoms in.
The cyanate ion is an anion composed of one oxygen atom one carbon atom and one nitrogen atom in the order OCN. Double bonds act as one electron pair to help determine electron-pair geometries of molecules according to VESPR. We review their content and use your feedback to keep the quality high.
100 1 rating Number of valence e. It possesses 1 unit of a negative charge borne by the nitrogen atom. Therefore the carbon is in the center bonding with the two sulfur atoms forming the bond angle.
What is the correct Lewis structure for cs2. A bonding orbital for Si1-N2 with 20000 electrons __has 2927 Si 1 character in a p-pi orbital 9804 p 196 d. If playback doesnt begin shortly try restarting your device.
Solution for n the lewis structure for the CS2 molecule the number of unshared pairs of electrons on the central carbon is. Lewis Structure of Dichlorine Monoxide OCl2 Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. Aluminum bromide is formed from the Al3 cation and the Br anion.
Which one of the following pure substances will exhibit hydrogen bonding. CS2 is an abbreviated form of Carbon Disulphide. Is there something else I should know for the purpose of answering a question like this on my test.
CS 2 is named Carbon Disulfide. There are 16 valence electrons available for the Lewis structure for CS 2. What is the electron geometry for CS2.
What is the formal charge on the central Cl atom. BrF3 CS2 SiF4 SO3.

Cs2 Molecular Geometry Shape And Bond Angles Youtube
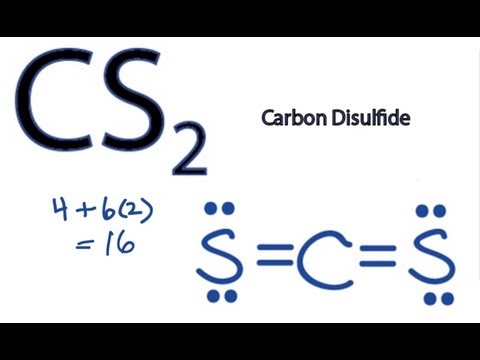
Cs2 Lewis Structure How To Discuss
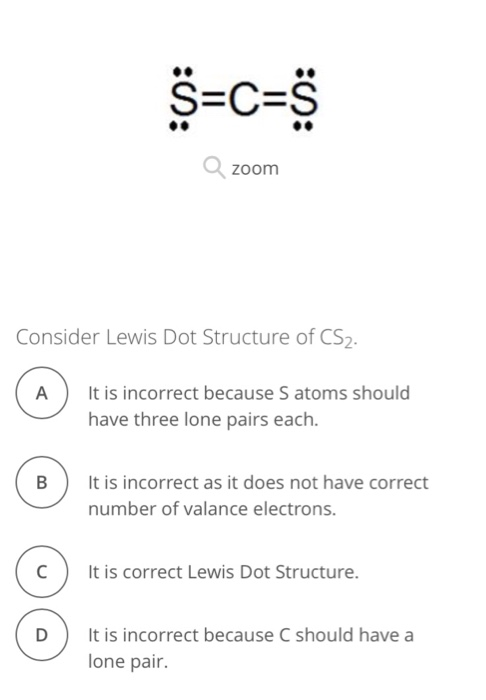
Solved S C S Zoom Consider Lewis Dot Structure Of Cs2 Ait Is Chegg Com

How To Draw Ch2cl2 Lewis Structure Science Education And Tutorials
Answer In General Chemistry For Kimmay17 256314

Solved Saved Identify The Correct Lewis Structure For Cs2 Chegg Com
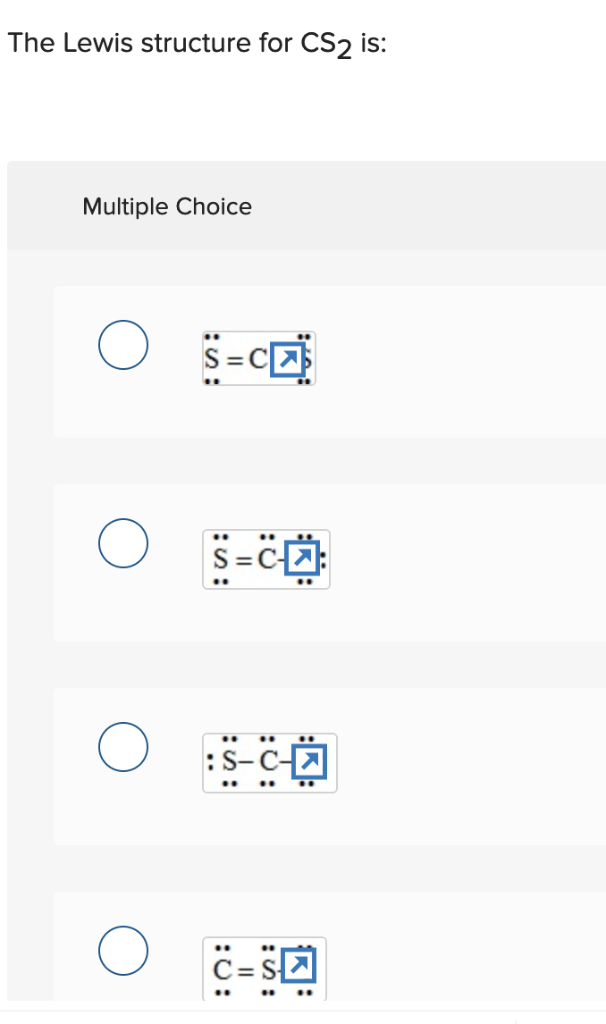
Solved The Lewis Structure For Cs2 Is Multiple Choice Chegg Com
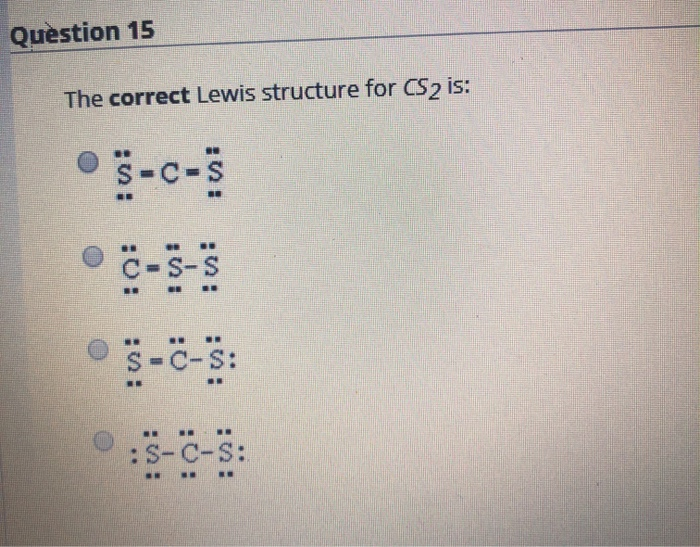
Solved Question 15 The Correct Lewis Structure For Cs2 Is Chegg Com

Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube

Cs2 Lewis Structure Carbon Disulfide Youtube

How To Draw The Lewis Structure Of Cs2 Carbon Disulfide Youtube

How Many Double Bonds Does Cs2 Have 2021 Practical Guide

Cs2 Lewis Structure Hybridization Molecular Shape And Polarity Techiescientist

How To Draw Cs2 Lewis Structure Science Education And Tutorials
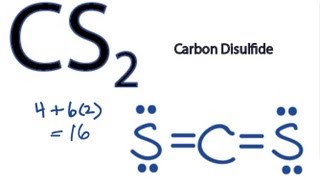
Cs2 Lewis Structure How To Draw The Lewis Structure For Cs2 Youtube
Cs2 Lewis Structure Hybridization Polarity And Molecular Shape
Cs2 Lewis Structure Hybridization Polarity And Molecular Shape

How To Draw Ash3 Lewis Structure Science Education And Tutorials
Cs2 Lewis Structure Hybridization Polarity And Molecular Shape

Comments
Post a Comment